Introduction to Teeth Whitening Techniques
Teeth whitening, a process designed to effectively brighten discolored teeth, has transformed into an efficient, economical, and minimally invasive cosmetic treatment. Introduced two decades ago by Haywood and Haymann, this at-home dental remedy has continuously proven to enhance the aesthetic appeal of stained teeth. Not only does whitening improve tooth color, but it also benefits periodontal health, promoting overall oral hygiene. Furthermore, the temporary stimulation of soft and hard tissues involved is considered harmless and reversible. In this article, we will explore various commonly used teeth whitening methods employed both in clinical settings and at home.
The Mechanism of Teeth Whitening
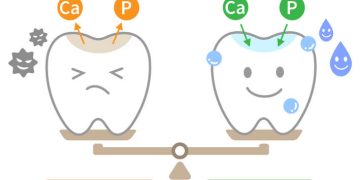
Teeth whitening can be accomplished through three primary processes: etching, abrasion, and oxidation. Among these, oxidation is the most widely embraced due to the potential irreversible alterations to tooth morphology associated with etching and abrasion. Excessive enamel loss resulting from these methods could inadvertently accentuate the darker underlying dentin. The oxidation process utilizes bleaching agents to penetrate the enamel matrix, transforming chromatic carbon rings into lighter carbon chains. The degree of whitening is proportional to the concentration of the bleaching agent used—higher concentrations yield quicker and more pronounced effects. This process modifies the enamel’s microstructure, impacting both its organic and inorganic phases. However, a notable downside is that bleaching agents with low pH can contribute to enamel demineralization. Luckily, this effect is often transient, as calcium present in saliva and the use of fluoride toothpaste can lead to the remineralization of the enamel surface.
Common Bleaching Agents and Techniques
The most frequently utilized bleaching agents include urea peroxide (1%–45%), hydrogen peroxide (3%–50%), and sodium perborate. These compounds can be formulated into a variety of applications, such as gels, pastes, powders, rinses, and toothpaste. The concentration of the bleaching agent directly dictates the duration of the treatment. Higher concentrations can yield rapid results, while lower concentrations necessitate extended application times. There are two principal methods of bleaching: in-office whitening and at-home whitening (often under professional supervision). The latter can be paired with an in-office “quick start” treatment, allowing for a more gradual completion at home.
Vital Tooth Bleaching
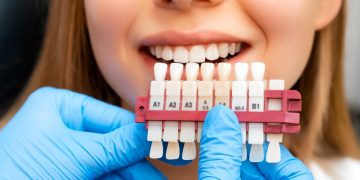
Vital tooth bleaching is specifically intended for lightening discolored teeth and may serve as a crucial component of aesthetic treatment plans, such as preparations for veneers, crowns, or white fillings. When employed independently, bleaching can address intrinsic stains appearing yellow, brown, gray, or attributable to tetracycline exposure. This technique involves creating a custom plastic tray using a heat/vacuum forming machine. The selected bleaching agent fills these trays, which are then placed over the teeth for a designated period, typically followed by brushing with high-concentration sodium fluoride (2800 ppm) toothpaste. The timeline for this treatment is contingent upon the extent of discoloration; for instance, daily applications of 10% urea peroxide for 2-3 hours may require 2-6 weeks to achieve desired results. Color improvements are measurable on the Vita Classic Shade Guide, with potential shifts of 2-10 shades in lightness. However, it is important to note that a rebound of 1-2 shades may occur 2-4 weeks post-treatment, prompting suggestions to allow some waiting period prior to subsequent cosmetic restorations. For severe discoloration, such as marked tetracycline staining, whitening may extend up to 6-12 months. Throughout the bleaching process, professional oversight is advisable, with the progress monitored via photographs and corresponding shade guides.
Non-Vital Tooth Bleaching
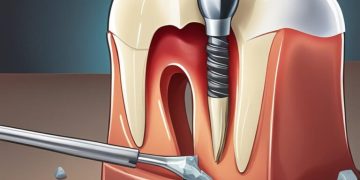
Non-vital tooth bleaching, commonly known as internal bleaching, is utilized to address discoloration following root canal therapy. This method is often referred to as crown internal bleaching or transitional bleaching. Prior to commencing, it is essential to ensure that existing root fillings provide satisfactory apical sealing to prevent bleaching agents from seeping into the periapical region, which could lead to root resorption. This technique involves placing the bleaching agent within the pulp chamber and sealing it with glass ionomer or resin-based composite to thwart bacterial leakage. Light-activated bleaching employs bleaching agents containing carotene, which are accelerated when exposed to high-intensity light or laser, enhancing the active component’s breakdown. Although light does not directly contribute to the whitening effect, the heat generated can enhance the bleaching agent’s efficacy. Moreover, the heat can dehydrate teeth, provoking increased sensitivity post-treatment.

Protective Measures in Bleaching
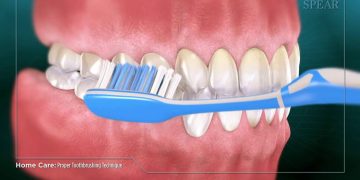
While effective and straightforward, specific prerequisites must be acknowledged before engaging in teeth whitening procedures. 1. Protecting the Teeth: Pre-treating sensitive areas, such as cervical regions or wedge-shaped defects, should include sealing with glass ionomer. Furthermore, whitening teeth with significant wear and exposed dentin may exacerbate dentin erosion. For those with visible fluorosis or developmental bands, micro-abrasion may be necessary to prevent uneven results. 2. Protecting the Gums: Utilization of rubber dams or other gingival barriers is vital when applying high-concentration bleaching gels in clinical settings.
Understanding the Side Effects of Teeth Whitening
While teeth whitening is generally considered safe, there are potential side effects that should be addressed. 1. Immediate reduction in enamel microhardness (25 microns of the outer layer) occurs after bleaching, typically restored within 3-4 weeks through salivary remineralization. 2. Over-bleaching at high concentrations may provoke chemical and morphological changes in enamel structure, leading to increased porosity, pitting, reduced fracture toughness, and early caries-like lesions. 3. Tooth and gum sensitivity can arise, particularly with high-concentration agents; yet, this can usually be mitigated with sodium fluoride, potassium nitrate, or sodium citrate to foster remineralization of the affected areas. 4. Reduced bonding strength with dentin (especially with sodium perborate), although bonding strength with enamel shows minimal change within a week post-bleaching. 5. Increased micro-leakage at the interface between dentin and restorative materials, though enamel margins remain intact. 6. Effects on restoration materials, particularly composite and glass ionomer fillings, may exacerbate visibility of existing restorations. 7. Cervical root resorption may also manifest.
Key Points of Bleaching
In conclusion, teeth whitening represents a straightforward, effective, and largely non-harmful method for achieving a brighter smile. Nevertheless, professional supervision and careful monitoring are essential in optimizing treatment outcomes and minimizing adverse effects.




























Discussion about this post