For decades, the discovery of antibiotics represented a monumental victory in medicine, transforming once-lethal infections into manageable conditions. In dentistry, antibiotics became a crucial tool for controlling severe oral bacteria, preventing the spread of infection after procedures, and safeguarding patients with specific health conditions. However, this era of confidence is waning. A silent but steady crisis is unfolding in dental clinics and hospitals worldwide: the rise of antibiotic-resistant oral infections. These are infections caused by bacteria that have evolved to survive the very drugs designed to kill them, turning standard treatments into ineffective remedies and transforming routine dental problems into potential life-threatening emergencies.
This growing threat challenges the foundational principles of modern dentistry and demands a paradigm shift in how we approach oral health. The question is no longer if we will encounter these superbugs, but how we can adapt to prevent and combat them. This article will investigate the complex causes fueling this resistance, explore the emerging alternatives to traditional antibiotic treatment, and underscore why impeccable dental hygiene has transitioned from a personal health goal to a critical public health responsibility in the age of resistance.
The Perfect Storm: Unpacking the Causes of Resistance
The development of antibiotic resistance is a natural evolutionary process, but human action has accelerated it into a global crisis. In the context of oral health, several factors converge to create a perfect storm.
- Indiscriminate and Inappropriate Prescribing: Historically, antibiotics have been overprescribed in dentistry. This includes:
- “Just in Case” Prescriptions: Prescribing antibiotics prophylactically for procedures with a low risk of infection, such as routine extractions in healthy patients.
- Treatment of Non-Bacterial Conditions: Prescribing antibiotics for toothaches caused by pulpitis (an inflammation of the nerve, not an infection) or viral conditions, where they have no effect.
- Patient Pressure: Prescribing antibiotics to meet patient expectations, even when not clinically indicated.
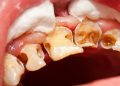
- The Unique Oral Environment: The mouth is a complex biological haven. It is home to a diverse microbiome, with hundreds of different bacterial species living in a biofilm—a sticky, protective community attached to surfaces like teeth and gums. This biofilm, especially in the form of plaque, acts as a physical barrier, making it difficult for antibiotics to penetrate and reach bacteria in effective concentrations. Within this protected environment, bacteria can easily share resistance genes with one another, even between different species, rapidly spreading the ability to withstand antibiotics.
- The Agricultural and Global Link: The widespread use of antibiotics in livestock for growth promotion and disease prevention contributes significantly to the reservoir of resistance genes. These resistant bacteria can enter the human population through the food chain, water, and the environment. Furthermore, in our interconnected world, a resistant strain developing in one part of the globe can quickly spread to another, making antibiotic resistance a borderless problem.
The Shifting Paradigm: Exploring Treatment Alternatives
With the efficacy of standard antibiotics diminishing, dentistry is being forced to innovate, returning to fundamental surgical principles and embracing advanced technologies.
- The Primacy of Source Control: The most effective way to treat a localized oral infection is to physically remove its source. This is the cornerstone of managing resistant infections. This involves:
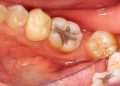
- Incision and Drainage (I&D): For abscesses, surgically opening the site to release the pus, which contains a high concentration of bacteria, immediately reduces the infectious load.
- Root Canal Treatment: Removing the infected nerve tissue from within a tooth and sealing the space.
- Tooth Extraction: Removing the source of the infection entirely.
- Culture and Sensitivity Testing: In the face of a persistent or severe infection that does not respond to initial treatment, the old approach of empirically prescribing a broad-spectrum antibiotic is becoming obsolete. The modern standard is to take a sample of the pus or tissue and send it to a lab. There, the specific bacteria are cultured and tested against a panel of antibiotics to identify which one, if any, will be effective. This targeted approach ensures the right drug is used, improving outcomes and slowing the development of further resistance.
- Advanced and Alternative Therapies: Research is intensifying into non-antibiotic solutions:
- Photodynamic Therapy (PDT): This involves applying a photosensitizing solution to the infected area and then exposing it to a specific wavelength of light. The light activates the solution, producing reactive oxygen species that kill bacteria without harming human cells and without promoting resistance.
- Silver Nanoparticles: Known for their broad-spectrum antimicrobial properties, silver nanoparticles are being incorporated into dental materials, coatings for implants, and even irrigation solutions to combat resistant biofilms.
- Bacteriophages: These are viruses that specifically infect and kill bacteria. Phage therapy represents a highly targeted approach that could be used to eradicate specific resistant pathogens.
The First and Last Line of Defense: The Paramount Importance of Dental Hygiene
In an era where our most powerful medicines are failing, prevention is not just better than cure—it is the only guaranteed cure. The importance of rigorous, daily dental hygiene has been elevated from a personal recommendation to a critical public health imperative.
Disrupting the Biofilm: The primary goal of effective oral hygiene is to mechanically disrupt the plaque biofilm before it matures into a pathogenic, organized community that can harbor resistant bacteria. Consistent brushing and flossing are the most powerful tools we have to prevent infections from starting in the first place.
Controlling the Oral Microbiome: A healthy, balanced oral microbiome, maintained through good hygiene, is more resilient and less likely to be dominated by disease-causing pathogens. By keeping bacterial numbers low and diversity high, we reduce the opportunities for resistant mutants to emerge and thrive.
Reducing the Need for Intervention: A cavity-free mouth with healthy gums significantly reduces the likelihood of needing invasive dental procedures that might have previously warranted a “just in case” antibiotic prescription. Every cavity prevented, and every case of gingivitis halted, is a direct blow against the selective pressure that drives antibiotic resistance.
Conclusion: A Collective Responsibility for a Post-Antibiotic Era
The rise of antibiotic-resistant oral infections is a stark warning and a call to action. It is a problem created by misuse and overuse, and it must be solved through stewardship, innovation, and a renewed commitment to prevention.
The responsibility is shared. Dentists must embrace their role as stewards of antibiotics, prescribing them judiciously and only when absolutely necessary, while prioritizing mechanical debridement. Researchers must continue to develop the next generation of targeted, non-antibiotic therapies. And for the public, the message has never been clearer: the daily, seemingly mundane acts of brushing with fluoride toothpaste, flossing meticulously, and attending regular dental check-ups are no longer just about avoiding a filling. They are a vital form of self-defense in a world where the miracle drugs of the past are losing their power. Our collective oral hygiene is now the bedrock of our defense against the silent pandemic of antimicrobial resistance.




























Discussion about this post