The allure of natural, affordable, and seemingly powerful home remedies is stronger than ever. In the quest for a whiter, brighter smile, many people turn to two common household staples: baking soda and hydrogen peroxide. Celebrated for their cleaning and bleaching properties in other contexts, they have become DIY dentistry favorites. Social media feeds are filled with videos promising dramatic whitening results from pastes and rinses made from these ingredients. But beneath the surface of these appealing promises lies a critical question: are these harsh chemicals secretly damaging your teeth in the name of beauty?
The answer is nuanced and hinges entirely on concentration, frequency, and application method. Used recklessly, these substances can cause irreversible harm. Used with knowledge and caution, they can have a place in a careful oral hygiene routine. This article will dissect the safety profile of each ingredient, delve into the hard science of their effects on tooth enamel, and provide clear, evidence-based guidelines for any potential proper application.
The Abrasive Truth: Baking Soda and Your Enamel
Baking soda, or sodium bicarbonate, is a mild alkaline compound with two primary dental properties: abrasiveness and stain-lifting ability.
The Safety and Efficacy Profile:
On the positive side, baking soda’s mild abrasiveness is effective at removing extrinsic stains—those on the surface of the teeth from coffee, tea, and red wine. Its alkaline nature can also help neutralize plaque acids, temporarily raising the pH in the mouth and creating an environment less hospitable to the bacteria that cause cavities.
However, the core risk lies in its Relative Dentin Abrasivity (RDA). The RDA scale measures how abrasive a toothpaste or substance is on dentin, the sensitive layer beneath the enamel. While enamel is the hardest substance in the human body, it is not indestructible.
- Water has an RDA of 0.
- The FDA considers a dentifrice with an RDA of under 250 to be safe for enamel.
- Most commercial toothpastes have an RDA between 60 and 100.
- Plain baking soda has an RDA of approximately 7.
At first glance, baking soda’s RDA of 7 seems very low and safe. However, this measurement is for its pure, powdered form. The danger arises when people mix it into a paste. To create a usable consistency, individuals often add too little water, creating a thick, gritty slurry. This concentrated paste is far more abrasive than the measured RDA suggests. Furthermore, people tend to scrub aggressively with a stiff-bristled brush, compounding the abrasive action.
The Enamel Effects:
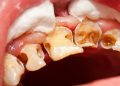
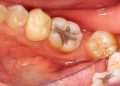
Enamel does not regenerate. Once it’s gone, it’s gone forever. The consistent use of a gritty baking soda paste, especially with aggressive brushing, acts like fine sandpaper on the teeth. It can lead to:
- Enamel Erosion: A gradual wearing away of the protective enamel layer.
- Dentin Exposure: As enamel thins, the underlying yellow dentin becomes visible, making teeth appear more yellow—the exact opposite of the desired effect.
- Tooth Sensitivity: Exposed dentin contains microscopic tubules that lead directly to the nerve, causing sharp pain with hot, cold, or sweet stimuli.
- Gum Recession and Abrasion: Harsh scrubbing can also damage the softer gum tissue, leading to recession and notches at the gumline.

The Bleaching Agent: Hydrogen Peroxide Under the Microscope
Hydrogen peroxide is a potent oxidizing agent and the active whitening ingredient in almost all professional and over-the-counter whitening products. Its safety and efficacy are entirely dependent on its concentration and the control of its application.
The Safety and Efficacy Profile:
At appropriate concentrations and in controlled settings, hydrogen peroxide is safe and effective. It works by penetrating the enamel and breaking down the complex, discolored molecules trapped inside the tooth (intrinsic stains) into smaller, colorless fragments, thereby lightening the overall tooth color.
Professional in-office whitening uses high concentrations (25-40%) for short periods under strict supervision, often with protective barriers for the gums. At-home whitening strips and trays from reputable brands use low concentrations (3-10%) in carefully designed applicators that minimize gum contact.
The danger of DIY hydrogen peroxide use stems from three factors:
- Unregulated Concentration: The hydrogen peroxide available at drugstores is typically a 3% solution, which is the standard for topical antiseptic use. While this is a low concentration, using it as a mouthwash without dilution or control is risky.
- Uncontrolled Application: Swishing with a hydrogen peroxide solution allows it to freely contact and soak into the soft tissues of the gums, tongue, and cheeks.
- Lack of pH Control: Professional whitening gels are formulated with a specific pH and contain desensitizing agents to protect the tooth. A straight hydrogen peroxide solution lacks these protective buffers.
The Enamel and Soft Tissue Effects:
The primary risk of hydrogen peroxide is not to the enamel itself, but to the living tissues of the mouth.
- Tooth Sensitivity: Just as with professional whitening, improper use can cause significant, temporary tooth sensitivity as the peroxide permeates the enamel and irritates the nerve.
- Chemical Burns on Gums: Concentrated or prolonged contact with hydrogen peroxide can cause a condition known as chemical gingivitis, where the gum tissue becomes white, inflamed, and sloughs off. This is a direct chemical burn that damages the mucosal lining.
- Cellular Toxicity: Studies have shown that high concentrations of hydrogen peroxide can be cytotoxic to the pulp (the living center of the tooth) and the cells of the oral mucosa, with potential long-term consequences that are still being studied.
The Path of Least Damage: Guidelines for Any Proper Application
Given the risks, the safest professional advice is to avoid DIY concoctions and use professionally formulated products. However, for those who insist on using these ingredients, strict guidelines must be followed to minimize harm.
For Baking Soda:
- Extreme Dilution: Mix a tiny amount (a pinch, no more than a quarter teaspoon) with a generous amount of water to create a very thin, non-gritty milky solution.
- Use as an Occasional Rinse, Not a Paste: Swish with this diluted solution for no more than 60 seconds to help neutralize acids after a meal. Do not use it as a brushing paste.
- If Used as a Paste (Not Recommended): If you must, use the highly diluted paste with an extra-soft toothbrush, apply zero pressure, and brush for no more than 30 seconds. Limit this to once a week at most.
- Always Follow with Fluoride: After any contact with baking soda, rinse thoroughly and then use a standard fluoride toothpaste to brush normally. This helps remineralize any microscopic scratches created by the abrasive.
For Hydrogen Peroxide:
- Dilution is Mandatory: If using a 3% solution, dilute it further with an equal part of water to create a 1.5% solution.
- Limit Contact Time: Swish for no more than 30-60 seconds.
- Avoid Swallowing: Be extremely careful not to swallow any of the solution.
- Rinse Thoroughly: Follow immediately with a clean water rinse.
- Frequency: Do not use a hydrogen peroxide rinse more than once or twice a week, and discontinue immediately if you experience any gum whitening, irritation, or heightened sensitivity.
Conclusion: A High-Risk, Questionable-Reward Endeavor
The central question—do these natural remedies damage teeth?—has a clear answer: Yes, they absolutely can, and the risk of irreversible damage is significant.
The perceived benefits of baking soda and hydrogen peroxide are overshadowed by the potential for enamel erosion, dentin exposure, severe tooth sensitivity, and chemical burns to the gums. The margin for error is slim, and the long-term cost of damaged teeth far outweighs the short-term savings of a DIY approach.
Modern dentistry offers a plethora of safe, effective, and regulated alternatives. For whitening, over-the-counter strips with controlled peroxide levels or professionally supervised treatments are the scientifically validated path. For stain removal, a soft-bristled brush and a toothpaste with a safe level of silica abrasives are all that is needed.
Ultimately, your enamel is a finite resource. Trusting its care to an unregulated, unpredictable home experiment is a gamble with poor odds. The safest and most effective oral care routine will always be one built on products that have been rigorously tested and approved by dental associations, used under the guidance of a dental professional.




























Discussion about this post